Which of the Following Best Describes Spectator Ions
The answer is b. They are called spectator ions because they are present.

How To Write The Net Ionic Equation For Ca No3 2 Na3po4 Ca3 Po4 2 Nano3 Youtube
That do not participate in the reaction B.

. Which of the following best describes a direct result of the common ion effect. Which of the following will be spectator ions in the reaction in aqueous solution of leadII nitrate and potassium chloride. D The both result in forming one.
Then identify the spectator ions. A net ionic equation shows only the chemical change that is taking place in a reaction. A principle that describes the relationship between the pressure and volume of a gas at constant temperature.
A student is given a sample in lab that contains one of. There are no spectator ions in this reaction. 1 mark Net ionic equation.
Which of the following correctly defines spectator ions. When the Ag and Cl- ions collide they will form an ionic bond which causes them to clump together and form a precipitate. A They both involve hydrogen.
Pb2 and NO3 e. Select all that apply. The Na and NO_3 - ions are present in the container where the reaction is occurring but they are not part of the solid product which precipitates.
The ion is unchanged on both sides of a chemical equation and does not affect equilibrium. The solubility rules apply to aqueous and non-aqueous solutions. Look for something that is the same on both sides and does not change state.
All soluble ionic compounds are considered strong electrolytes. HgBra is more soluble in a solution of KBr than in pure water. NaNO3 is more soluble in.
Which of the following is a spectator ion in the following reaction. Select one or more. HgBrą is less soluble in a solution of KBr than in pure water.
2 marks Total ionic equation. You just studied 32 terms. Ions that combine to form an insoluble precipitate.
NisPb2Ni2Pbs For the following chemical equation. Lons made when compounds dissolve in water C. Which of the following statements correctly describe a net ionic equation.
The nitrate ions are spectator ions since they are on both the reactant and the product side of the equation and can be removed from the equation giving us the following. The solubility rules apply only to ionic substances. All species are represented as ions in this type of equation.
See the answer See the answer done loading. Spectator Ion Definition. Ions that appear in a net ionic equations.
C They both result in splitting a molecule. Ions that do not participate in the reaction although they are present. Which is a spectator ion from the following complete ionic equation.
Ions that are dissociated in solutions. Which statement BEST describes a similarity between a hydration and a dehydration reaction. Usually insoluble salts will become more soluble if the solution contains ligands.
Therefore these ions are the spectator ions. This is a spectator ion and can be crossed out of the net ionic equation. K and NO3.
Up to 24 cash back Which of the following best describes the term end point. Which of the following concerning electrolytes and the solubility rules isare true. B They both involve addition to a double bond.
When writing a net ionic equation spectator ions found in the original equation are ignored. 1 mark Spectator ions. Check all that apply.
Which of the following will be spectator ions in the reaction in aqueous solution of leadII nitrate and potassium chloride. If you write out a normal equation. A net ionic equation consists of the formulas for the spectator ions.
Pb2 K Cl and NO3 d. That are made of a single element D. In the reaction between HNO₃ and BaOH₂ assume complete reaction of the BaOH₂ the spectator ions are H aq OH aq H₂O l Which one of the following represents the net ionic equation for the reaction of HBr with CaOH₂.
Spectator ions may be either cations positively-charged ions or anions negatively-charged ions. Ions that combine to form a precipitate. So the spectator ions for the given reaction are NO3 and NH4 answer choice C.
A spectator ion is an ion that does not take part in the reaction. A titration is performed on a 250 mL sample of calcium hydroxide with a 015 molL solution of nitric acid. Now up your study game with Learn mode.
Which of the following best describes spectator ions. They are Na Cl- Ag and NO_3 - ions. Pb2 and C1 O bKand CI c.
Sodium and sulfate are spectator ions or tribuned ions because they do not react the other two will give precipitated MgOH2. 2Na s 2H2O l 2NaOH aq H2 g Na.

Acid Base Neutralization Reactions Net Ionic Equations Chemistry Youtube

Solved What Is A Net Ionic Equation What Species Are Shown In Such An Equation And Which Species Are Not Shown
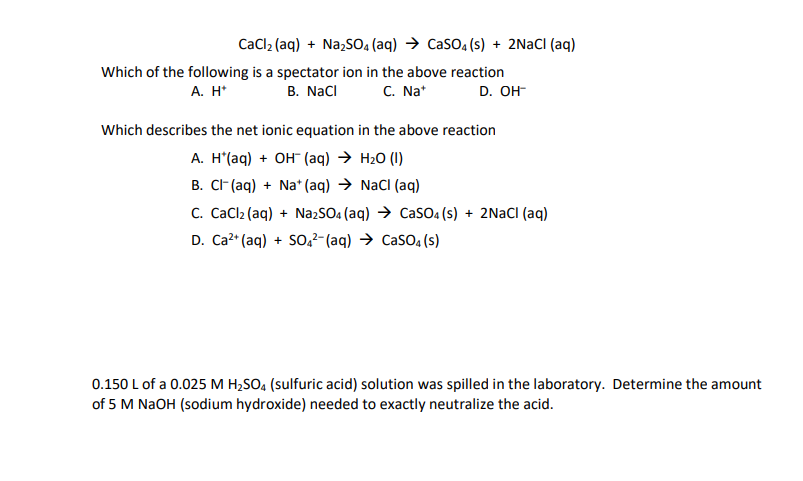
Solved Cacl2 Aq Na2so4 Aq Casou S 2nacl Aq Chegg Com
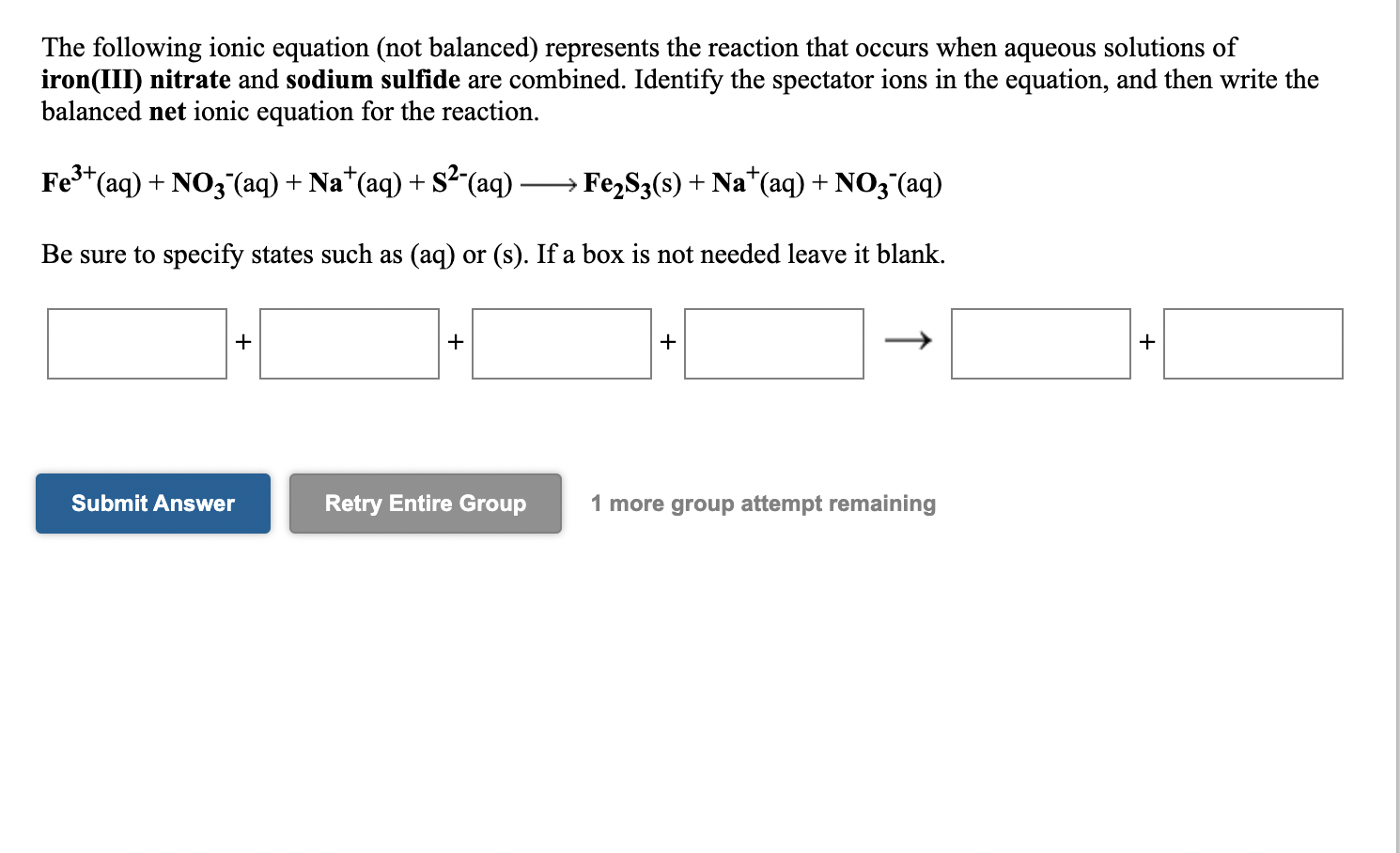
Solved The Following Ionic Equation Not Balanced Chegg Com

Answered Write A Balanced Net Ionic Equation For Bartleby

Solved Identify The Spectator Ions In The Following Chegg Com

Solved Which Is Are The Spectator Ion S In The Following Chegg Com

Solved Question 1 Identify The Spectator Ions In The Chegg Com
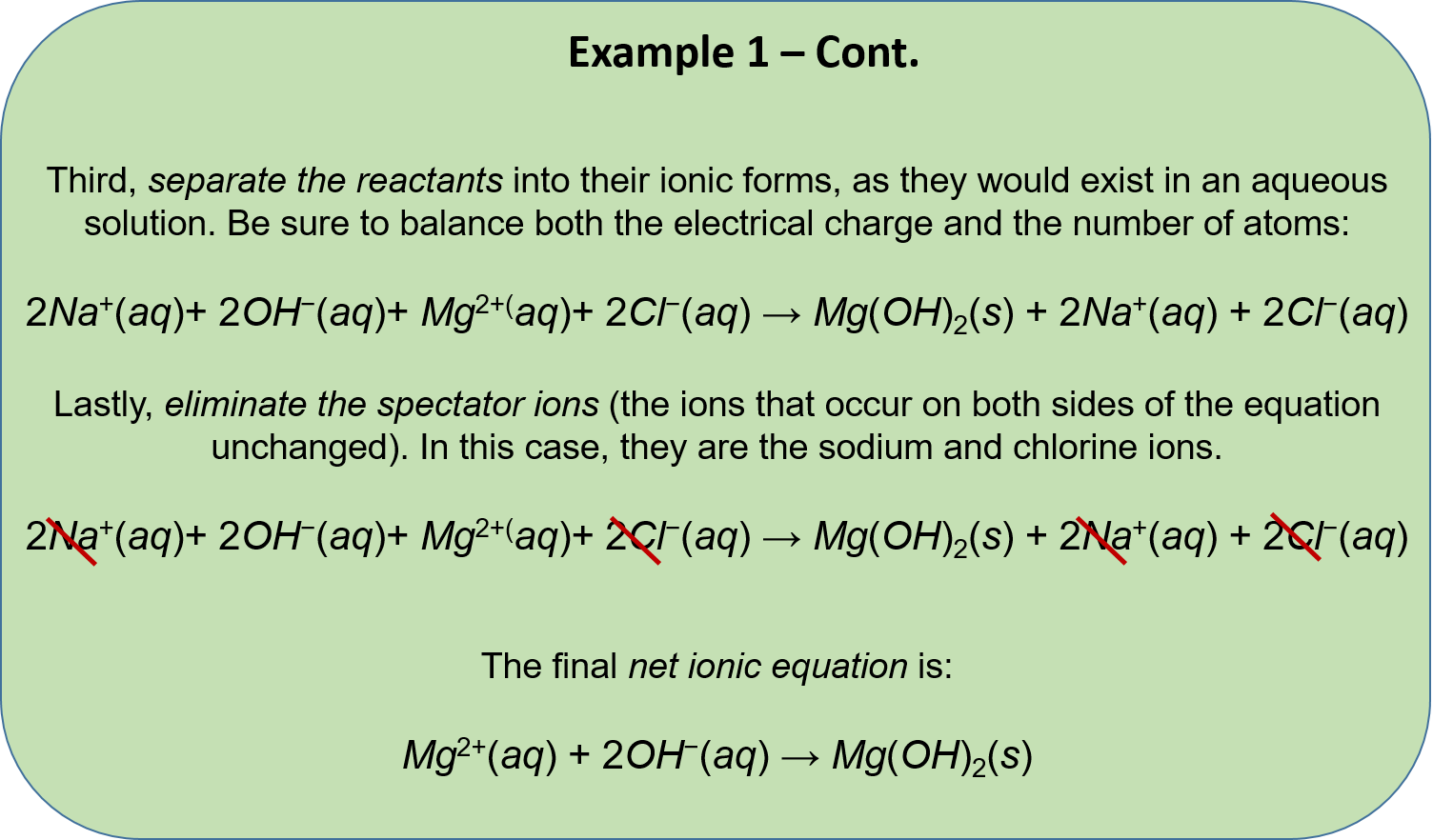
Ch104 Chapter 5 Chemical Reactions Chemistry

What Spectator Ions Are And Why They Are Important Periodic Table Words How To Memorize Things Chemical Equation
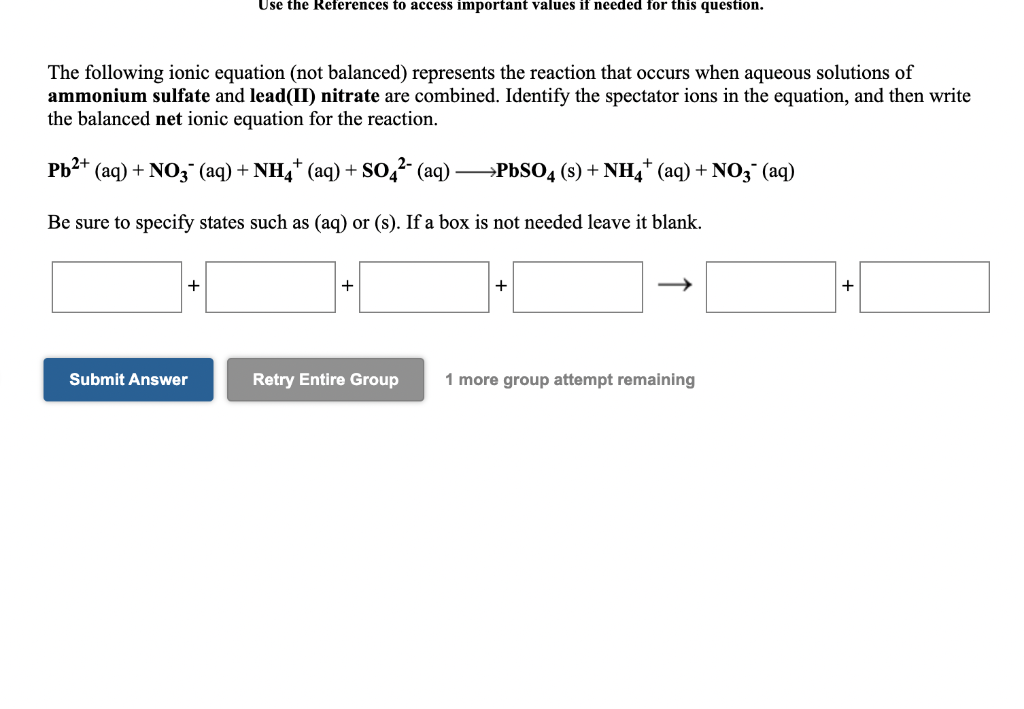
Solved The Following Ionic Equation Not Balanced Chegg Com
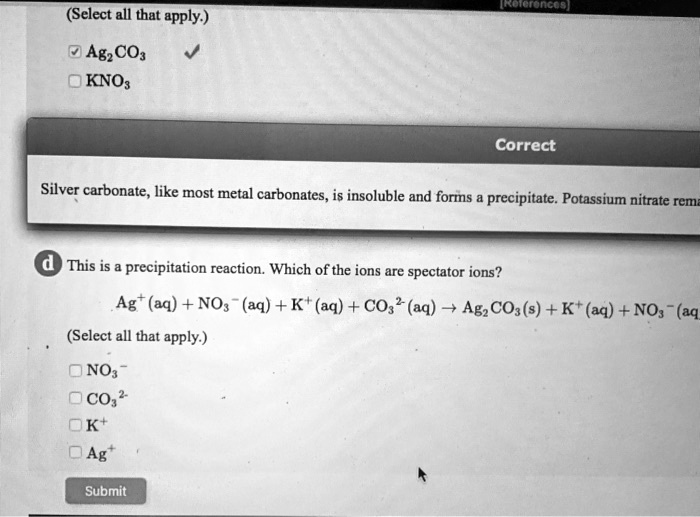
Solved Select All That Apply Agzcos Kno Correct Silver Carbonate Like Most Metal Carbonates Is Insoluble And Forms Precipitate Potassium Nitrate Rem This Is Precipitation Reaction Which Of The Ions Are Spectator Ions

How To Write The Net Ionic Equation For Na2s Pb No3 2 Nano3 Pbs Youtube
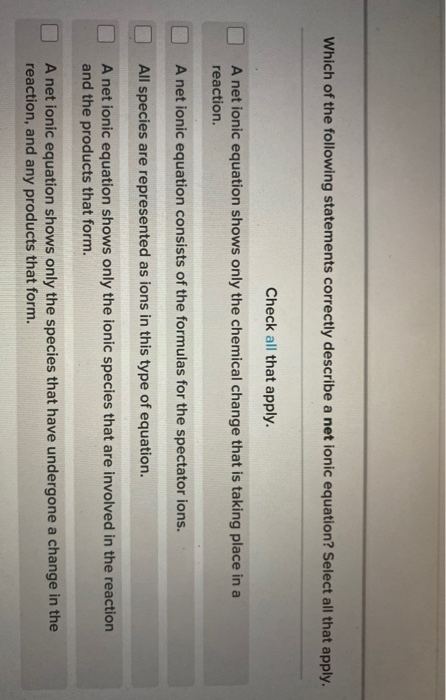
Solved Which Of The Following Statements Correctly Describe Chegg Com
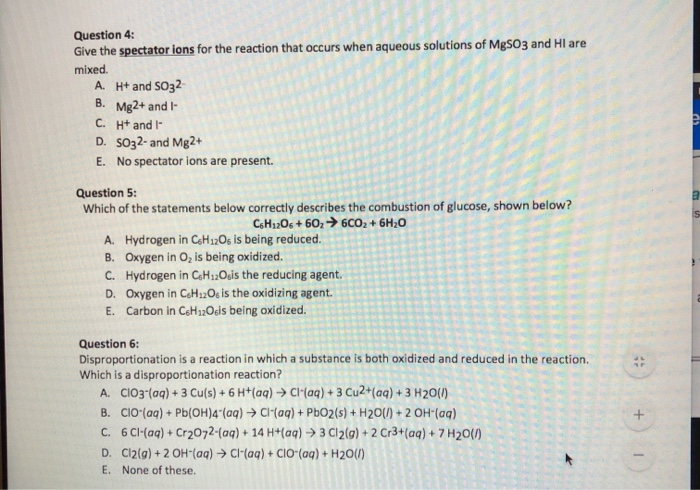
Solved Question 4 Give The Spectator Ions For The Reaction Chegg Com

Solved Question 4 Give The Spectator Ions For The Reaction Chegg Com
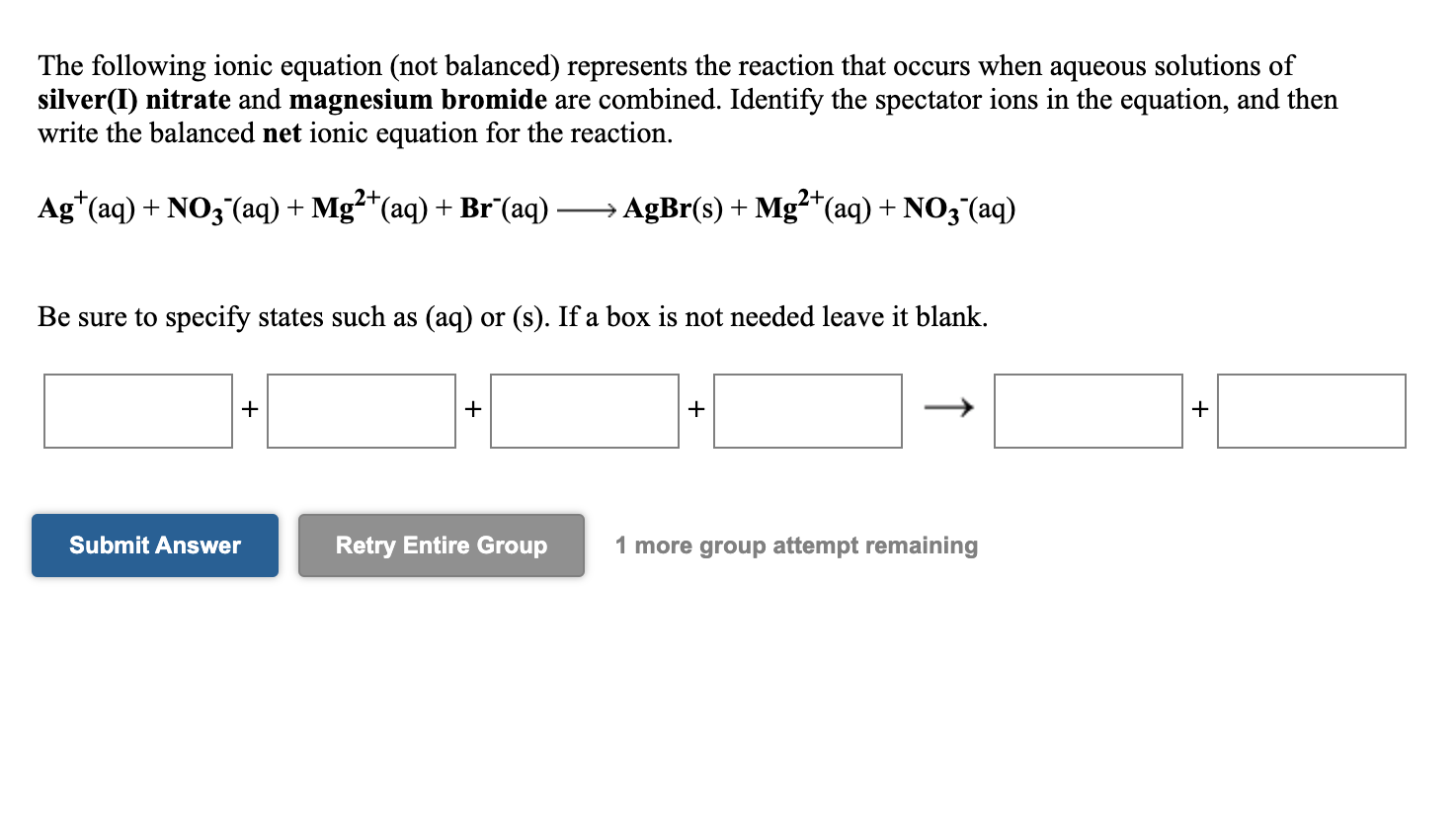
Solved The Following Ionic Equation Not Balanced Chegg Com
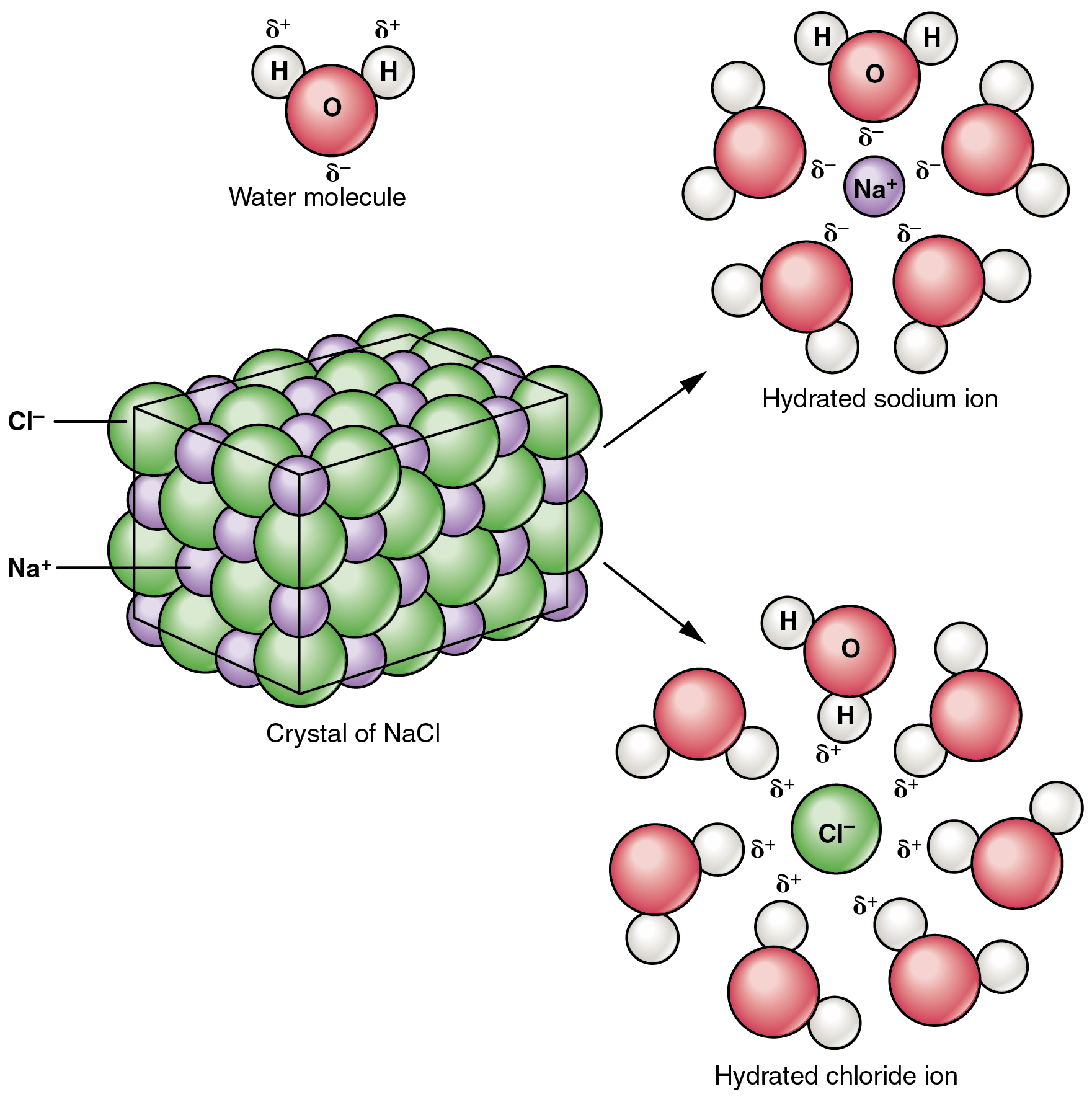
Molecular Complete Ionic And Net Ionic Equations Article Khan Academy

Comments
Post a Comment